Sinopharm COVID-19 Vaccine: 5 Things to Know | Health Plus
03 Aug 2021
After the Sinovac vaccine, the Sinopharm BBIBP-CorV vaccine has now been approved for use by private clinics in Singapore against the COVID-19 virus under the Special Access Route. Here are 5 things you should know about the Sinopharm vaccine.
Last updated on 18 January 2022
1. The Sinopharm vaccine is the first China-made vaccine to be approved by the World Health Organisation (WHO)
The Sinopharm COVID-19 vaccine, BBIBP-CorV was approved for use by the China’s National Medical Products Administration in December 2020 and gained WHO’s approval for emergency use in May 2021.
The vaccine was developed by the Beijing Bio-Institute of Biological Products (BBIBP) and is the first China-made COVID-19 vaccine approved by the WHO for emergency use. It is also the first approved COVID-19 vaccine developed by a non-Western country. Sinovac’s vaccine, the CoronaVac, is the second.
To date, more than a billion doses of the Sinopharm BBIBP-CorV have been administered. The vaccine has also been approved for use in over 50 countries around the world. It is currently undergoing Phase 3 trials in Middle Eastern countries such as Egypt and the United Arab Emirates (UAE), and South American countries like Argentina and Peru.
The BBIBP-CorV is a two-dose vaccine, with a recommended gap of 3 weeks between the doses. Similar to the Sinovac vaccine, it is recommended for individuals aged 18 years and older who have no history of anaphylaxis to any component of the vaccine. Further research is needed to demonstrate its efficacy against severe COVID-19 disease in persons with other diseases, pregnant women and individuals aged above 60 years old. However, no upper age limit has been set for the vaccine as it is likely to have a protective effect in older adults and is unlikely to differ in safety for both older and younger adults.
2. Both the Sinopharm BBIBP-CorV and Sinovac’s CoronaVac vaccines are inactivated vaccines
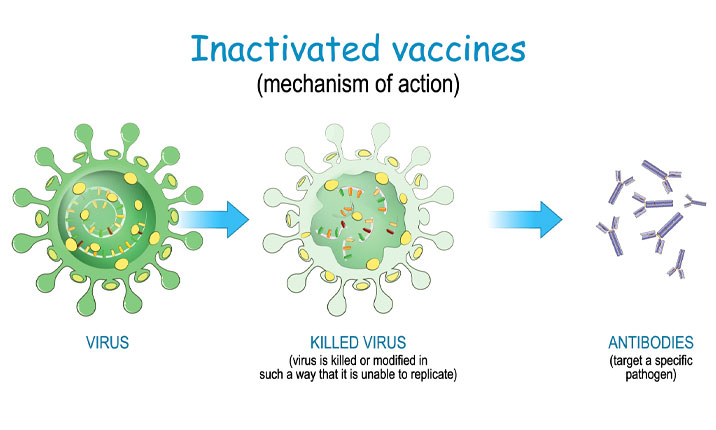
Unlike the Pfizer-BioNTech/Cominarty and Moderna vaccines which use mRNA technology, the Chinese-made vaccines are inactivated vaccines which use killed COVID-19 viral particles. In these vaccines, the surface spike protein of the virus particle is kept intact to trigger the body’s immune system to develop antibodies to protect against the live COVID-19 virus in the event that the person is infected.
Inactivated vaccines are a tried-and-tested form of vaccine technology which is used in other well-established vaccines, such as the flu and hepatitis B vaccines.
3. Sinopharm’s efficacy: How it compares with the Sinovac CoronaVac and Pfizer-BioNTech/Cominarty COVID-19 vaccines
Sinopharm’s COVID-19 vaccine proved 79% effective in preventing symptomatic infection in the multi-country Phase 3 trial. This is compared to 51% for Sinovac’s and 95% for the Pfizer-BioNTech/Cominarty COVID-19 vaccine.
More studies are required to prove the BBIBP-CorV’s efficacy against COVID-19 variants.
4. Most reported side-effects of the Sinopharm vaccine were mild to moderate
The most common side-effects of the Sinopharm vaccine are pain at the injection site, headache, and fatigue. However, 2 serious side effects may possibly be linked to the vaccine – serious nausea, and acute disseminated encephalomyelities, a neurological disorder involving inflammation of the brain and spinal cord.
5. It has much easier storage requirements compared to the mRNA vaccines
Due to the fragility of the mRNA molecule, mRNA vaccines need to be stored in sub-zero temperatures in the long-term. In comparison, both the Sinopharm and Sinopharm vaccines can be stored at a refrigerator temperature of 2 – 8 degree Celsius. This makes shipment and storage of these vaccines less costly and highly suitable for countries with low resources. The Sinopharm vaccine is also the first vaccine that will carry a vaccine vial monitor, which is a sticker that changes colour when the vaccine is exposed to heat. This allows for closer monitoring of whether the vaccine is safe to use.
Register your interest for the Sinopharm vaccine
If you have yet to be vaccinated against COVID-19, it is important for you to receive a COVID-19 vaccine as soon as you can to protect yourself against developing severe COVID-19 infection. The benefits of receiving approved COVID-19 vaccines outweigh the risks.
The Sinopharm vaccine will be made available soon to interested individuals at Parkway Shenton clinics. You may register your interest with us to be kept informed once the vaccine is made available. Do discuss your suitability for the Sinopharm vaccine with your doctor if you intend to take the vaccine.
Article reviewed by Dr Edwin Chng, medical director at Parkway Shenton, One Raffles Quay
References
Christopher, M. (2020, July 27). Acute Disseminated Encephalomyelitis (ADEM). Retrieved June 28, 2021, from https://www.webmd.com/brain/acute-disseminated-encephalomyelitis-adem
Cornwell, A., & Ghantous, G. (2021, June 4). (OFFICIAL) UAE, BAHRAIN make Pfizer/BioNTech shot available to those who GOT Sinopharm VACCINE (1419773954 1033280411 B. Berkrot, Ed.). Retrieved June 28, 2021, from https://www.reuters.com/world/middle-east/uae-bahrain-make-pfizerbiontech-shot-available-those-who-got-sinopharm-vaccine-2021-06-03/
COVID-19 Vaccine Storage Solution. (n.d.). Retrieved July 29, 2021, from https://www.melingbiomedical.com/en/solutions/vaccine-storage-solution/
Evidence Assessment: Sinopharm/BBIBP COVID-19 vaccine (Rep.). (2021, April 29). Retrieved June 28, 2021, from World Health Organisation website: https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/2_sage29apr2021_critical-evidence_sinopharm.pdf?sfvrsn=3dfe32c1_5
Hart, J., & Russell, F. (2021, June 21). What are the Sinopharm and Sinovac vaccines? And how effective are they? Two experts explain. Retrieved June 28, 2021, from https://theconversation.com/what-are-the-sinopharm-and-sinovac-vaccines-and-how-effective-are-they-two-experts-explain-162258
Interim recommendations for use of the Pfizer–BioNTech COVID-19 vaccine, BNT162b2, under Emergency Use Listing (Rep.). (2021, June 15). Retrieved June 28, 2021, from World Health Organization website: Interim recommendations for use of the Pfizer–BioNTech COVID-19 vaccine, BNT162b2, under Emergency Use Listing
Pike, H. (2021, May 18). Sinopharm COVID-19 vaccine: Should you worry about the side effects? Retrieved June 28, 2021, from https://www.medicalnewstoday.com/articles/sinopharm-covid-19-vaccine-should-you-worry-about-the-side-effects
Sinopharm (Beijing): BBIBP-CorV. (n.d.). Retrieved June 28, 2021, from https://covid19.trackvaccines.org/vaccines/5/
The Sinopharm Covid-19 vaccine: What you need to know. (2021, May 10). Retrieved June 28, 2021, from https://www.who.int/news-room/feature-stories/detail/the-sinopharm-covid-19-vaccine-what-you-need-to-know
Sinopharm: Chinese Covid vaccine gets WHO emergency approval. (2021, May 07). Retrieved June 28, 2021, from https://www.bbc.com/news/world-asia-china-56967973
Sinovac COVID-19 Vaccine: All you need to know. (2021, June 22). Retrieved June 28, 2021, from https://www.parkwayshenton.com/healthplus/article/sinovac-covid-vaccine-safety-efficacy-side-effects
Tan, Y. (2021, January 14). Covid: What do we know about China's coronavirus vaccines? Retrieved June 28, 2021, from https://www.bbc.com/news/world-asia-china-55212787
WHO lists additional COVID-19 vaccine for emergency use and issues interim policy recommendations. (2021, May 7). Retrieved July 29, 2021, from https://www.who.int/news/item/07-05-2021-who-lists-additional-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations




